completeFT™
Full Thickness Placental
Membrane

completeFT™ is a dehydrated three-layer placental allograft that may be used as a protective barrier in wound care applications, to include non-healing wounds and surgically created wounds.
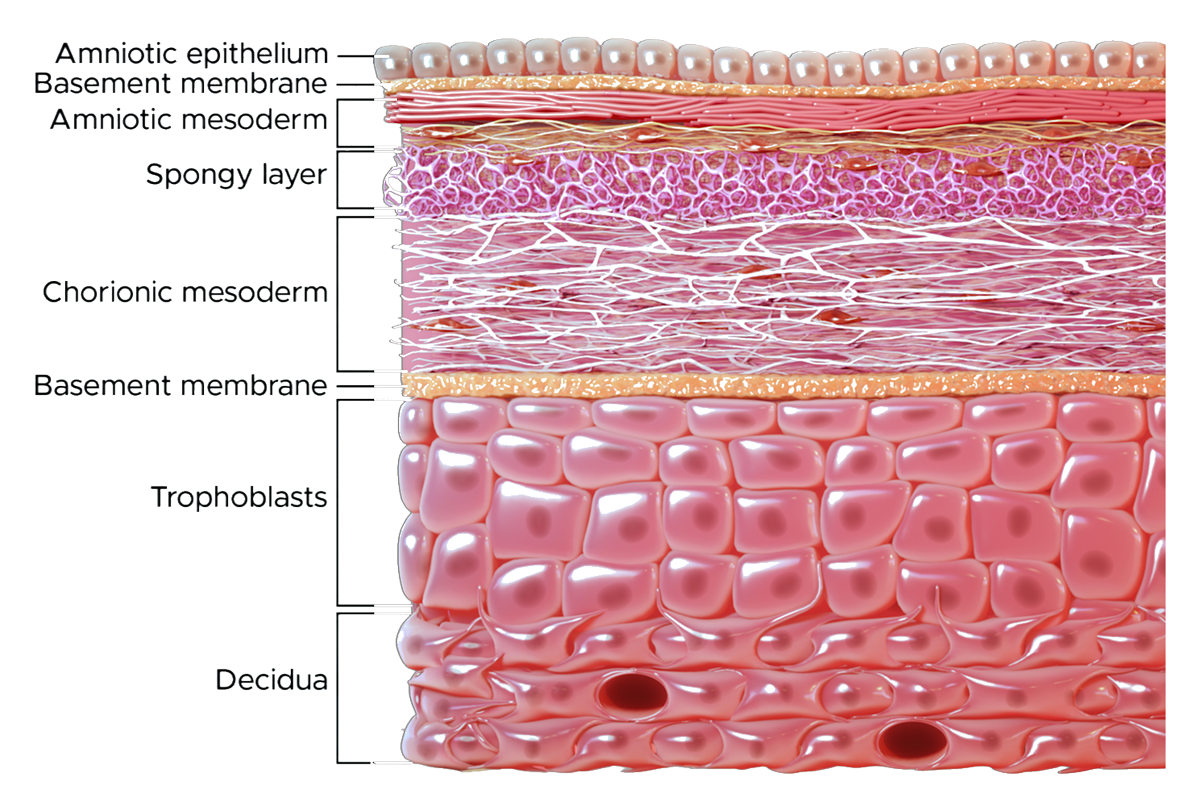
- Contains placental tissue in full thickness composition that includes the amnion, intermediate spongy layer, and chorion, which are key placental structural components retained to allow the membrane its utility to serve as a barrier.
- Processed using proprietary technology that retains the original relevant characteristics of native placental tissue.
- Intended for homologous use only. May act as a wound cover that is a natural barrier that shields wounds from the external environment.
- Is terminally sterilized via gamma irradiation and stored at ambient temperature with a 5-year shelf life.
- HCPCS: Q4271
Amniotic tissue allografts are not intended to diagnose, treat, cure or prevent any disease.
NOTE: The FDA’s Tissue Reference Group (TRG) has noted that completeFT™ appears to meet all of the criteria for regulation solely under section 361 of the Public Health Service Act and the regulations in 21 CFR part 1271 governing Human Cell, Tissue and Cellular and Tissue-Based Products (HCT/Ps).
Ordering Information
| SKU | Description | Size | Unit | UPC |
|---|---|---|---|---|
| EFT1515 | completeFT™ Placental Allograft Membrane | 1.5x1.5cm | 3 | 382567002209 |
| SKU | EFT1515 | |||
| Description | completeFT™ Placental Allograft Membrane | |||
| Size | 1.5x1.5cm | |||
| Unit | 3 | |||
| UPC | 382567002209 | |||
| EFT22 | completeFT™ Placental Allograft Membrane | 2x2cm | 4 | 382567000311 |
| SKU | EFT22 | |||
| Description | completeFT™ Placental Allograft Membrane | |||
| Size | 2x2cm | |||
| Unit | 4 | |||
| UPC | 382567000311 | |||
| EFT24 | completeFT™ Placental Allograft Membrane | 2x4cm | 8 | 382567000328 |
| SKU | EFT24 | |||
| Description | completeFT™ Placental Allograft Membrane | |||
| Size | 2x4cm | |||
| Unit | 8 | |||
| UPC | 382567000328 | |||
| EFT44 | completeFT™ Placental Allograft Membrane | 4x4cm | 16 | 382567000335 |
| SKU | EFT44 | |||
| Description | completeFT™ Placental Allograft Membrane | |||
| Size | 4x4cm | |||
| Unit | 16 | |||
| UPC | 382567000335 | |||
| EFT48 | completeFT™ Placental Allograft Membrane | 4x8cm | 32 | 382567000359 |
| SKU | EFT48 | |||
| Description | completeFT™ Placental Allograft Membrane | |||
| Size | 4x8cm | |||
| Unit | 32 | |||
| UPC | 382567000359 |
